Research at EyeCare Partners
Advancing the Field of Eye Care, One Clinical Trial at a Time
EyeCare Partners is committed to innovation and elevating eye care for everyone. ECP is home to world-renowned centers of eye care excellence and leaders from every specialty and subspecialty. The eye doctors and research staff at ECP have been conducting clinical research for more than 35 years and have participated in over 500 research studies. Our goal is to pave the way for tomorrow’s eye care and bring it to our patients today.
The EyeCare Partners practices participate in numerous studies across all subspecialties, these include dry eye, glaucoma, cataracts, macular degeneration, diabetes and more. Explore our current clinical research studies with open enrollment and complete the form to learn more about taking part.

How Does Clinical Research Help Our Patients?
Thousands of patients with eye problems come to an EyeCare Partners practice each week to find leading-edge solutions to their eye care needs. Our participation in clinical research allows us to offer patients the most advanced treatment options possible.

Staying on the Leading Edge of Eye Care Research
Our ongoing commitment to physician-controlled eye care research enables us to:
- Provide patients with new and improved treatment options
- Be at the forefront of vision care advances
- Create an environment for lifelong learning and advancement
- Advance the future of eye health and vision
Clinical Study Sponsors
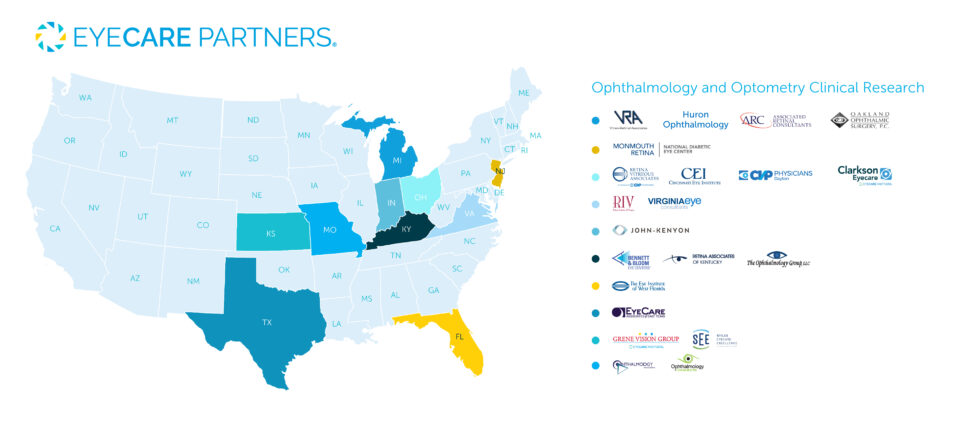
EyeCare Partners plays an active role in clinical research to advance the future of eye care. Our experienced eye doctors and specialized research teams partner with emerging and established pharmaceutical and biotechnology companies to explore the next generation of ocular treatments. EyeCare Partners has 20+ practices and over 100 Investigators that participate in more than 200 clinical trials and we look forward to continuing our commitment to state-of-the-art clinical research.
What Type of Clinical Research Happens at EyeCare Partners?
Our doctors are always seeking solutions for eye conditions and vision issues. The clinical trials at EyeCare Partners spans Phases 1-4 and a range of specialties. Examples of research topics include:
New and Innovative Ocular Medications
- Dry Eye Medications and Treatments
- Ocular Surface Disease
- Glaucoma Medications
- Wet and Dry Macular Degeneration
- Diabetic Retinopathy
- Diabetic Macular Edema
- Inherited Retinal Disease
- Uveitis
Novel Surgical Procedures, Devices, and Intraocular Implants
- Intraocular Lenses for Cataract Surgery
- Micro-Invasive Glaucoma Devices
- Specialized Corneal Transplantation
- Artificial Iris Devices
- Diabetic Macular Edema
- Wet Macular Degeneration

Meet Our Director of Clinical Research
Megan Kingdon, BSN, RN, COA, CCRC, Director of Clinical Research – EyeCare Partners
Megan Kingdon, Director of Clinical Research at EyeCare Partners, brings more than 20 years of expertise in ophthalmology and clinical research. She spent many of those years at the Cincinnati Eye Institute (CEI), starting as an ophthalmic technician and quickly moving into a clinical research coordinator role in Anterior Segment. She obtained her nursing degree and did a stent in obstetrics at UC Hospital before returning to CEI as the clinical research coordinator for the retina department, where she grew the department from three clinical trials to thirty active trials. In 2021, she became the Director of Clinical Research for CEI Vision Partners in the Retina Department and then following the acquisition by EyeCare Partners, she was made Director of Clinical Research for ECP, overseeing more than 20 practices across the country.
Megan has extensive medical and surgical clinical trial experience in a wide range of ophthalmological conditions, including cataracts and IOLs, artificial iris devices, age-related macular degeneration, diabetic retinopathy, geographic atrophy, macular edema, proliferative vitreo-retinopathy, retinal vein occlusion, vitreomacular traction and inherited retinal diseases. Megan holds a Bachelor’s of Science in biology and chemistry from Lawrence University and a Bachelor’s of Science in nursing from the University of Cincinnati. She is certified by the Association of Clinical Research Professionals.
Why Choose EyeCare Partners For Clinical Research?
Facilities
- State-of-the-art imaging and diagnostic equipment
- Dedicated, visual acuity testing rooms
- Designated monitoring rooms with high speed internet and phone access
Staff
- Experienced and award-winning eye doctors
- Dedicated clinical research coordinators
- Certified Ophthalmic Technicians and Photographers
- Trained personnel for handling/preparing for transportation of dangerous goods
Process
- Adherence to Good Clinical Practice guidelines
- Customized standard operating procedures
- Excellent subject retention
- Direct oversight by the PI and Research Director
Current Clinical Research Studies
Kansas
Stiles EyeCare Excellence
Abbvie
Study to Evaluate the Safety and Effectiveness of the XEN 63 Glaucoma Gel Stent Using Ab Interno and Ab Externo Implantation Approaches
Alcon
THE HYDRUS® MICROSTENT NEW ENROLLMENT POST-APPROVAL STUDY: A PROSPECTIVE, NON-RANDOMIZED, MULTICENTER, SINGLE ARM, CLINICAL TRIAL (CONFIRM)
Glaukos
A Prospective, Multicenter Device Study in Subjects with Primary Open-Angle Glaucoma Who Have Failed Previous Medical and Surgical Treatment
Sanoculis
An Observer-Masked, Single-Arm, Multicenter Study to Evaluate the Safety and Effectiveness of Minimally Invasive Micro Sclerostomy (MIMS®) to Reduce Intraocular Pressure in Open-Angle Glaucoma Which Is Not Controlled Despite Polypharmacy
Grene Vision Group
KOWA
A Double-Masked, Randomized, Placebo-Controlled, Parallel-Group, 12-Week Administration With Two-Week Gradual Dose Taper Phase and 38-Week Follow-Up Phase, Phase 3 Study to Investigate the Safety and Efficacy of Ripasudil (K-321) Eye Drops After Simultaneous Cataract
Missouri
Ophthalmology Associates
Combangio/Kala
A Study to Evaluate the Safety and Efficacy of KPI-012 Ophthalmic Solution in Patients with Persistent Corneal Epithelial Defect (PCED)
CXL Ophthalmics
A Multicenter, Randomized, Double-Masked, Sham-Controlled, Parallel-Group Phase 3 Study to Evaluate Efficacy and Safety of Epithelium Corneal Crosslinking in Patients Aged 8-45 with Keratoconus
Ophthalmology Consultants
Rayner
Clinical Trial to Investigate the Safety and Effectiveness of a Hydrophilic Trifocal Intraocular Lens RAO603F in Comparison with a Monofocal Intraocular Lens (RAO600C)
Texas
EyeCare Associates of East Texas
Not Enrolling Trials Currently
Florida
Eye Institute of West Florida
Eyepoint
A Phase 3, Multicenter, Prospective, Randomized, Double-Masked, Parallel-Group Study of EYP-1901, a Tyrosine Kinase Inhibitor (TKI), Compared to Aflibercept in Subjects with Wet AMD
Virginia
Retina Institute of Virginia
RegenxBio
A Randomized, Partially Masked, Controlled, Phase 3 Clinical Study to Evaluate the Efficacy and Safety of RGX-314 Gene Therapy in Participants with nAMD.
EyeBio
A Randomized, Double-Masked, Multicenter, 3-Arm Pivotal Phase 2/3 Study to Evaluate the Efficacy and Safety of Intravitreal EYE103 Compared with Intravitreal Ranibizumab (0.5mg) in Participants with Diabetic Macular Edema
Genentech
A Phase I/II Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of RO7446603 Administered Alone or in Combination with Aflibercept or Faricimab in Patients with Diabetic Macular Edema
Virginia Eye Consultants
CXL Ophthalmics
A Multicenter, Randomized, Double-Masked, Sham-Controlled, Parallel-Group Phase 3 Study to Evaluate Efficacy and Safety of Epithelium Corneal Crosslinking in Patients Aged 8-45 with Keratoconus
Combangio/Kala
A Study to Evaluate the Safety and Efficacy of KPI-012 Ophthalmic Solution in Patients with Persistent Corneal Epithelial Defect (PCED)
Eyepoint
A Phase 3, Multicenter, Prospective, Randomized, Double-Masked, Parallel-Group Study of EYP-1901, a Tyrosine Kinase Inhibitor (TKI), Compared to Aflibercept in Subjects with Wet AMD
Kentucky
Retina Associates of Kentucky
Eyepoint
A Phase 3, Multicenter, Prospective, Randomized, Double-Masked, Parallel-Group Study of EYP-1901, a Tyrosine Kinase Inhibitor (TKI), Compared to Aflibercept in Subjects with Wet AMD
Annexon
A Phase 3, Multicenter, Randomized, Parallel-Group, Double-Masked, 2-Arm, Sham-Controlled Study of the Efficacy, Safety, and Tolerability of ANX007 Administered by Intravitreal Injection in Patients with Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration (AMD) – ARCHER II
4D Molecular Therapeutics
A Phase 3 Randomized, Double-Masked, Active-Controlled Trial of a Single Intravitreal Injection of 4D-150 in Adults with Macular Neovascularization Secondary to Age-Related Macular Degeneration (4FRONT-1)
Bennett and Bloom
Jaeb Center for Health Research
Diabetes Endothelial Keratoplasty Study (DEKS): Impact of Diabetes on Corneal Transplant Success and Endothelial Cell Loss
Indiana
John Kenyon
Not Enrolling Trials Currently
Ohio
Cincinnati Eye Institute
RegenxBio
A Randomized, Partially Masked, Controlled, Phase 2b/3 Clinical Study to Evaluate the Efficacy & Safety of RGX-314 Gene Therapy in Participants with nAMD
A Randomized, Partially Masked, Controlled, Phase 3 Clinical Study to Evaluate the Efficacy and Safety of RGX-314 Gene Therapy in Participants with nAMD
Glaukos
A Prospective, Multicenter Study of the Glaukos® iStent Infinite Trabecular Micro-Bypass System Model IS3 in Subjects with Mild to Moderate Primary Open-Angle Glaucoma
Genentech
A Phase IIa Multicenter, Open-Label, Single-Arm Study to Optimize Subretinal Surgical Delivery and to Evaluate Safety and Activity of Opregen in Patients with Geographic Atrophy Secondary to Age-Related Macular Degeneration
Beacon
A Phase 2/3, Randomized, Controlled, Masked, Multi-Center Study to Evaluate the Efficacy, Safety, and Tolerability of Two Doses of AGTC-501, a Recombinant Adeno-Associated Virus Vector Expressing RPGR (rAAV2tYF-GRK1-RPGR), Compared to an Untreated Control Group in Male Participants
Ascidian
Open-Label, Single Ascending Dose Study to Evaluate the Safety, Tolerability, and Preliminary Efficacy of Subretinal ACDN-01 in Participants with ABCA4-Related Retinopathy
Genentech
A Phase IIIb/IV, Multicenter, Visual Assessor Masked Study of the Efficacy and Safety of the Port Delivery System with Ranibizumab in Patients with Neovascular Age-Related Macular Degeneration Previously Treated with Intravitreal Agents Other than Ranibizumab
CXL Ophthalmics
A Multicenter, Randomized, Double-Masked, Sham-Controlled, Parallel-Group Phase 3 Study to Evaluate Efficacy and Safety of Epithelium Corneal Crosslinking in Patients Aged 8-45 with Keratoconus
KOWA
A Double-Masked, Randomized, Placebo-Controlled, Parallel-Group, 12-Week Administration With Two-Week Gradual Dose Taper Phase and 38-Week Follow-Up Phase, Phase 3 Study to Investigate the Safety and Efficacy of Ripasudil (K-321) Eye Drops After Simultaneous Cataract
Neuracle
A Phase 1/2a Open-Label Study to Evaluate Safety, Tolerability, and Preliminary Efficacy of NG101 AAV Gene Therapy in Subjects with Wet Age-Related Macular Degeneration
Regeneron
A Multicenter, Randomized, Double-Masked, Placebo-Controlled Phase 3 Study of the Efficacy, Safety, and Tolerability of Subcutaneously Administered Pozelimab in Combination with Cemdisiran or Cemdisiran Alone in Patients with Geographic Atrophy Secondary to Age-Related Macular Degeneration (AMD)
Regeneron
A Phase 3b, Single-Arm Study of Aflibercept 8 mg Dosed Every 4 Weeks in Adult Participants With Neovascular Age-Related Macular Degeneration (nAMD) or Diabetic Macular Edema (DME) (ELARA)
Ocular Therapeutix
A Phase 3, Multicenter, Double-Masked, Randomized, Parallel Group Study to Evaluate the Efficacy and Safety of Intravitreal OTX-TKI (Axitinib Implant) in Subjects with Neovascular Age-Related Macular Degeneration
Genentech
A Phase I/II Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of RO7446603 Administered Alone or in Combination with Aflibercept or Faricimab in Patients with Diabetic Macular Edema
CVP Physicians Dayton
Not Enrolling Trials Currently
Retina Vitreous Associates
Not Enrolling Trials Currently
Michigan
Associated Retinal Consultants
RegenxBio
A Randomized, Partially Masked, Controlled, Phase 2b/3 Clinical Study to Evaluate the Efficacy & Safety of RGX-314 Gene Therapy in Participants with nAMD
A Randomized, Partially Masked, Controlled, Phase 3 Clinical Study to Evaluate the Efficacy and Safety of RGX-314 Gene Therapy in Participants with nAMD
Ocular Therapeutix
A Phase 3, Multicenter, Double-Masked, Randomized, Parallel Group Study to Evaluate the Efficacy and Safety of Intravitreal OTX-TKI (axitinib implant) in Subjects with Neovascular Age-Related Macular Degeneration
Lowry Medical Research Institute
MacTel NHOR: A Natural History Observation and Registry Study of Macular Telangiectasia Type 2
Genentech
A Phase III, Multicenter, Randomized, Double-Masked, Sham-Controlled Study to Investigate the Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of R7200220 Administered Intravitreally in Patients with Uveitic Macular Edema.
A Phase IIIb/IV, Multicenter, Visual Assessor Masked Study of the Efficacy and Safety of the Port Delivery System with Ranibizumab in Patients with Neovascular Age-Related Macular Degeneration Previously Treated with Intravitreal Agents Other than Ranibizumab.
A Three-Part, Phase I Study to Investigate the Safety, Tolerability, Pharmacokinetics, and Efficacy of RO7250284 Following Intravitreal Administration of Multiple Ascending Doses and Continuous Delivery from the Port Delivery System in Patients with Neovascular Age-Related Macular Degeneration (BURGUNDY).
Kyowa Kirin
Phase II Clinical Studies of KHK4951 (Tivozanib Eye-Drop). One Study is for Patients with Neovascular Age-Related Macular Degeneration (nAMD) and the Other Study is for Diabetic Macular Edema (DME).
Oakland Ophthalmic Surgery
Apthera
IC-8 Apthera IOL New Enrollment Post Approval Study
Samsara
A prospective, multicenter clinical study of the Implantable Miniature Telescope, Model SING in patients with central vision impairment associated with end-stage age-related macular degeneration (AMD).
Huron
Spyglass
A Prospective, Multicenter, Randomized, Masked, Controlled Study to Evaluate the Safety, Efficacy, and Dose-Response of the Bimatoprost Implant System (39 mcg, 78 mcg) Used in Combination with the SpyGlass IOL Compared to Timolol Maleate Ophthalmic Solution, USP, 0.5%.